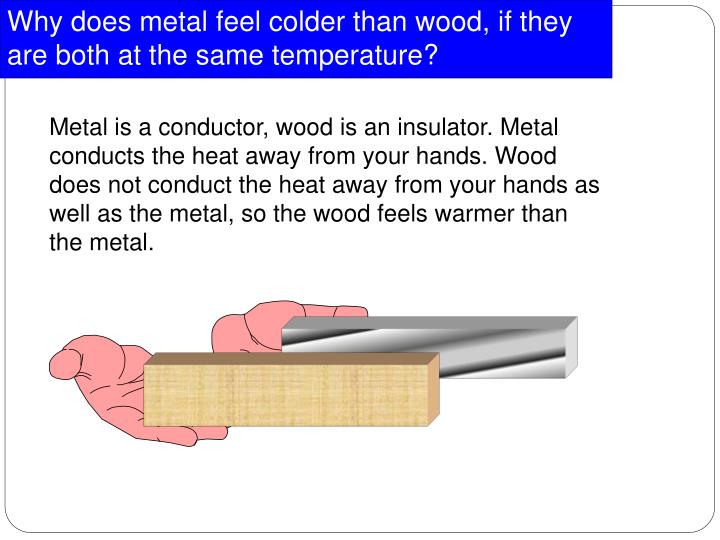
Does Metal Conduct Heat . learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. Learn the factors that determine the heat. metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. Find out why silver, copper and aluminum. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the.
from www.slideserve.com
learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. Find out why silver, copper and aluminum. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. Learn the factors that determine the heat.
PPT Conduction, Convection and Radiation PowerPoint Presentation ID
Does Metal Conduct Heat thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. Learn the factors that determine the heat. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. Find out why silver, copper and aluminum. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. metals are good conductors of heat and electricity because they have many free electrons that can move and collide with.
From courses.lumenlearning.com
Conduction Physics Does Metal Conduct Heat learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. Learn the factors that determine the heat. Find out why silver, copper and aluminum. metals are good conductors of heat and electricity because. Does Metal Conduct Heat.
From www.learnatnoon.com
Conduction of Heat in Metals and NonMetals Noon Academy Does Metal Conduct Heat Learn the factors that determine the heat. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another. Does Metal Conduct Heat.
From www.slideserve.com
PPT Science Fair Project PowerPoint Presentation ID708758 Does Metal Conduct Heat yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. Learn the factors that determine the heat. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. Find out why silver, copper and aluminum. thermal conductivity, the ability of a substance to conduct heat. Does Metal Conduct Heat.
From mavink.com
Conduction Diagram Heat Transfer Does Metal Conduct Heat thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. Learn the factors that determine the heat. yes, metals conduct heat. Does Metal Conduct Heat.
From www.slideserve.com
PPT AQA GCSE 1a1 Heat Transfer PowerPoint Presentation ID281065 Does Metal Conduct Heat Learn the factors that determine the heat. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. yes, metals conduct heat. Does Metal Conduct Heat.
From www.universetoday.com
The Science of Heat Transfer What Is Conduction? Universe Today Does Metal Conduct Heat Learn the factors that determine the heat. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. learn why metals generally conduct heat better than other solids,. Does Metal Conduct Heat.
From www.slideshare.net
Chapter 24 Conduction Does Metal Conduct Heat metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. learn why metals are good conductors of electricity and heat based. Does Metal Conduct Heat.
From www.pinterest.com
Metal definition An element that is a good conductor of heat and Does Metal Conduct Heat learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. learn how the number, mobility. Does Metal Conduct Heat.
From techiescientist.com
Does Metal Conduct Heat? (Detailed Explanation) Techiescientist Does Metal Conduct Heat yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. Find out why silver, copper and aluminum. Learn the factors that determine the heat. metals are good conductors of heat and electricity because they have. Does Metal Conduct Heat.
From www.slideserve.com
PPT Conduction, Convection and Radiation PowerPoint Presentation ID Does Metal Conduct Heat learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. thermal conductivity, the. Does Metal Conduct Heat.
From www.slideserve.com
PPT KS3 Physics PowerPoint Presentation, free download ID589588 Does Metal Conduct Heat learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. Learn the factors that determine the heat. Find out why silver, copper and aluminum. yes, metals conduct heat well. Does Metal Conduct Heat.
From www.ency123.com
Why are metals good conductors of heat and electricity? Does Metal Conduct Heat learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal. Does Metal Conduct Heat.
From telgurus.co.uk
How do metals conduct electricity? Chemistry Questions TEL Gurus Does Metal Conduct Heat Find out why silver, copper and aluminum. yes, metals conduct heat well because of their free electrons that facilitate thermal transfer. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat. Does Metal Conduct Heat.
From studyzonereflating.z13.web.core.windows.net
Metals That Conduct Heat Does Metal Conduct Heat Find out why silver, copper and aluminum. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. metals are good conductors of heat and electricity because they have. Does Metal Conduct Heat.
From vitzmetals.com
Does Steel Conduct Heat? Everything You Need to Know Does Metal Conduct Heat learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. Find out why silver, copper and aluminum. metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. Learn the factors that determine the heat. thermal conductivity, the. Does Metal Conduct Heat.
From brainly.in
Why do metals conduct heat? Brainly.in Does Metal Conduct Heat learn how the number, mobility and purity of free electrons influence a metal's ability to conduct heat and electric current. Find out why silver, copper and aluminum. Learn the factors that determine the heat. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. yes, metals conduct heat well. Does Metal Conduct Heat.
From www.slideshare.net
CHAPTER FIFTEEN Transmission of Heat Energy Does Metal Conduct Heat metals are good conductors of heat and electricity because they have many free electrons that can move and collide with. learn why metals generally conduct heat better than other solids, and how factors like electrical conductivity, crystal structure, and. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons.. Does Metal Conduct Heat.
From slideplayer.com
Ch. 5 Atoms and Bonding Section 4. Bonding in Metals ppt download Does Metal Conduct Heat Learn the factors that determine the heat. learn why metals are good conductors of electricity and heat based on their metallic bonding and free electrons. thermal conductivity, the ability of a substance to conduct heat or move heat from one location to another without the movement of the material conducting the. metals are good conductors of heat. Does Metal Conduct Heat.